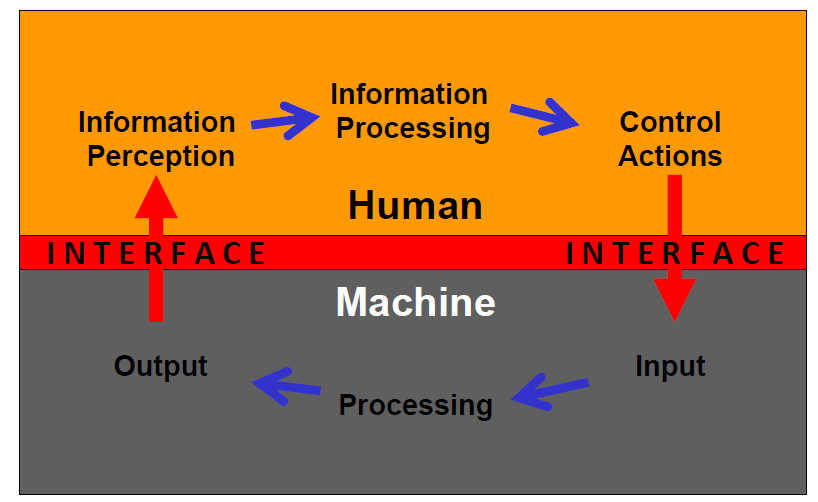
What is Usability File?
Term ‘usability` is “a multi-dimensional quality that refers to the ability of a human to interact easily and relatively error-free with a system or product.” Therefore, throughout time, terms such as ‘user-friendly’ or ‘intuitive’ have become connected to the concept of usability. It also indicates that a system should work the way users expect it to. When it comes to healthcare and medical devices, usability has a great impact on it. If a medical device is lacking usability, there is a higher possibility that errors occur, or the use of the device might be slower, i.e. not as intended. Consequently, delivery of therapy may suffer or lack completely and patient safety may be in danger.
Understanding Regulatory Requirements for Human Factors Usability Testing
What are human factors & usability?
Human factors: “…the application of knowledge about human capabilities (physical, sensory, emotional, and intellectual) and limitations to the design and development of tools, devices, systems, environments, and organizations….”
Usability: “Characteristic of the USER INTERFACE that establishes EFFECTIVENESS, EFFICIENCY, ease of USER learning and USER satisfaction” (ISO/IEC 62366:2007).
Why is FDA Concerned about HF?
- Device safety
- Device effectiveness
Ergonomics and “Hedonomics”
Device-User Interface
Device use Errors
For Example
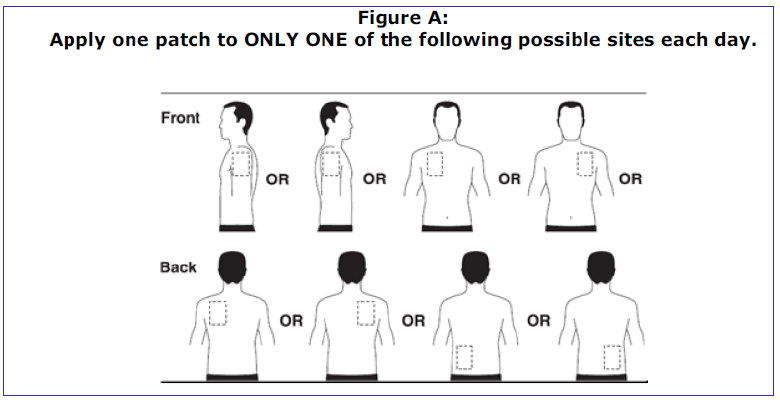
Transdermal Patch Products:
- Original user instructions: where to apply patch
- Revised user instructions: where to apply patch
FDA Regulations Relevant to HF
Quality System regulation:
- Design Controls -The need for human factors is implied:
- Design input – includes “needs of the user and patient”
- Design verification – performance criteria met
- Design validation – “… devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions. Design validation shall include software validation and risk analysis….” [incl. use‐related risks].
Design Input
User and Patient Needs
Users can operate the device without injury or negative clinical consequences to either the user or the patient
Relative to device effectiveness
Users can operate the device successfully for the intended uses
- Potential use errors and failures have been eliminated or limited to the extent possible through appropriate application of human factors methods
Design Verification:
Did I make the product right?
Design Validation:
Did I make the right product?
Human Factors Standards
AAMI/ANSI HE75:2009
General considerations and principles
- Managing the risk of use error
- Usability testing
Design elements
- Controls
- Software
Integrated solutions
- Mobile medical devices
- Home health care
ISO/IEC 62366:2007
Medical devices – Application of usability engineering to medical devices
- Usability engineering process
- Accompanying document
- Training
Note: Currently undergoing revision
ANSI/AAMI/ISO 14971:2007
Medical devices – Application of risk management to medical devices
- Risk management
- Risk analysis
- Risk evaluation
- Evaluation of overall residual risk acceptability